Viral Vector & Plasmid DNA Manufacturing Market by Type (Viral Vector (Retroviruses, Adenoviruses, AAV, Lentiviruses), Plasmid DNA), Workflow (Upstream, Downstream), Application (Cell & Gene Therapy), Diseases (Cancer), End User - Global Forecast to 2028
Market Growth Outlook Summary
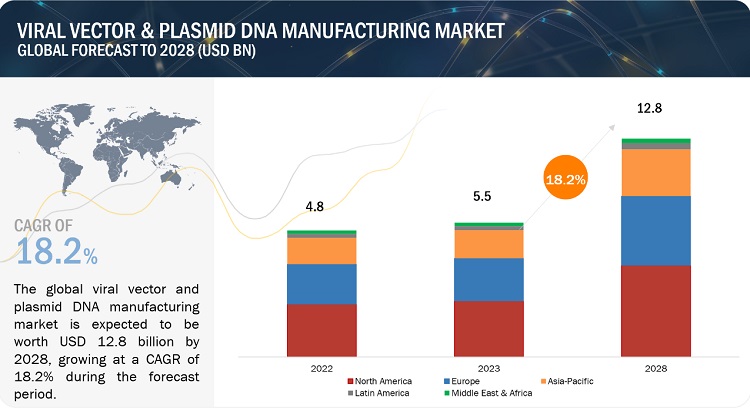
The global viral vector manufacturing market, valued at US$4.8 billion in 2022, stood at US$5.5 billion in 2023 and is projected to advance at a resilient CAGR of 18.2% from 2023 to 2028, culminating in a forecasted valuation of US$12.8 billion by the end of the period. The growth of this market is majorly driven by g rising prevalence of target diseases and disorders, the availability of funding for gene therapy development, and effectiveness of viral vectors in gene therapy delivery. On the other hand, high operational cost associated with cell & gene therapy manufacturing is restraining the growth of this market.
To know about the assumptions considered for the study, Request for Free Sample Report
Viral Vector Manufacturing Market
Driver: Effectiveness of viral vectors
Viral vectors have been proven to be effective tools for delivering therapeutic genes to target cells in gene therapy. The effectiveness of viral vectors can depend on several factors, including the type of virus used, the target cell type, the dosage and mode of delivery, and the immune response of the patient. Currently, viral vectors are the most effective gene delivery methods, especially for in vivo gene transfers. Viruses have a natural tropism to some cell types and are hence utilized in therapeutic approaches. Viral vectors can be modified according to the required application and thus prove advantageous for targeting and entering cells.
Restraint: High operational costs associated with cell & gene therapy manufacturing
Currently, more than 1,000 cell & gene therapies are in trial worldwide. There are more than 700 investigational cell & gene therapies in clinical development in the US alone. However, manufacturing facilities have not kept up. It has been estimated that hundreds of facilities will be needed to manufacture the treatments that are now in clinical trials. One of the areas that need to be accelerated is viral capacity. Most viral vectors are produced using adherent manufacturing, which is expensive to operate—a vial of 20 million cells can cost USD 20,000 to USD 30,000 to make. The cost of manufacturing for a gene therapy can be between USD 500,000 and USD 1 million, excluding the costs for R&D, the costs to run crucial clinical trials, or the costs to build the commercial infrastructure necessary to provide access to patients.
The cost of manufacturing a cell & gene therapy is significantly more than conventional biologics such as monoclonal antibodies and recombinant proteins. Therefore, the significant operational costs associated with cell and gene therapy manufacturing are expected to negatively impact the viral vector and plasmid DNA manufacturing market during the forecast period.
Opportunity: Leveraging digital to facilitate operational excellence
To further address operational challenges in production, manufacturers could explore the use of digital tools. One such tool is advanced analytics-based models, which can help optimize yield by proactively identifying and correcting potential yield issues. By using an analytics-driven recommendation engine built on historic deviation and root-cause data, manufacturers can shorten timelines for resolving deviations.
Another potential solution to the industry-wide talent shortage is the use of augmented and virtual reality tools. These tools can be used to train operators without removing the lab or subject-matter expert from production. They can be particularly useful for initial onboarding or infrequent processes that require refresher training. By leveraging these digital tools, manufacturers can improve efficiency and address challenges in production, ultimately leading to more successful outcomes in manufacturing of viral vectors and plasmid DNA.
Thus, increasing usage of digital and automation tools in manufacturing of viral vector and plasmid DNA is expected to drive the market growth over the forecast period.
Challenge: Risk of mutagenesis and other unwanted outcomes
Several safety concerns have been highlighted in viral vectors that are used for cell and gene therapy manufacturing. These include inflammation, random insertion disrupting normal genes, the activation of proto-oncogenes, and insertional mutagenesis. As several factors are associated with the risk of viral vector-mediated insertional mutagenesis (viral and non-viral), a one-size-fits-all approach for reducing genotoxicity cannot be applied. Current genotoxicity testing strategies rely largely on detecting the effects on DNA (damage or mutation) following a short exposure and expression period for mutation. However, these are of limited use when considering vector-mediated insertional mutagenesis, as it takes weeks, months, or even years to manifest in patients.
The choice of test models to detect toxicity is also vital. However, the development of effective test systems to detect viral vector-induced genotoxicity, particularly considering the delayed onset, represents a major challenge. New in vitro and in vivo assays are being developed for the assessment of non-viral vector risk factors, such as age and disease state. These are expected to improve the pre-clinical safety assessment of viral vectors and improve patient-centric risk assessment.
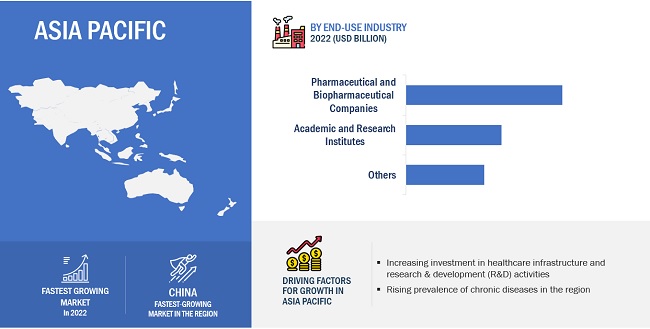
The products segment dominated viral vector and plasmid DNA manufacturing market by products & services
The products segment held the largest share of the global market in 2022. The process of viral vector and plasmid DNA manufacturing involves several key products and reagents that are essential for the successful production, purification, and characterization of viral vectors and plasmid DNA. Products considered under this segment are cell lines, specialized media, purification kits and reagent, assay kits, vector amplification kits and expansion products. The large share of this segment can be attributed to factors such as the increasing demand for viral vector and plasmid DNA based therapies, rising research and development activities are expected to rise the demand for products used in viral vector and plasmid DNA manufacturing and anticipated to drive the segment growth over the forecast period.
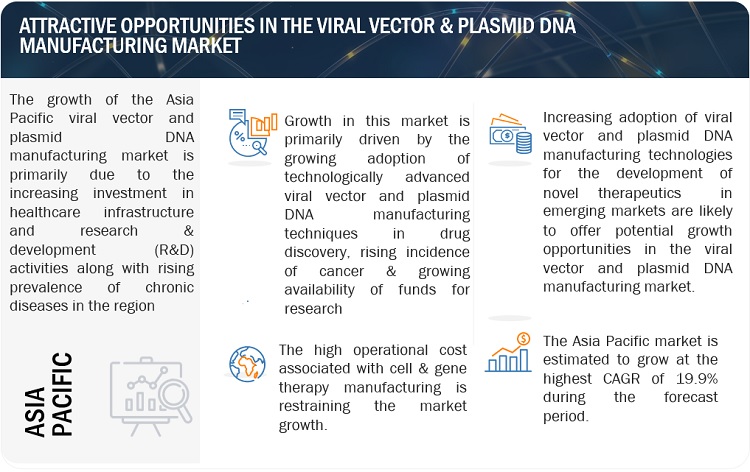
Asia Pacific region of viral vector and plasmid DNA manufacturing market is estimated to register the highest CAGR during the forecast period.
Asia Pacific offers lucrative growth potential for the market. This can be attributed to the increasing pharmaceutical R&D spending, the growing trend of outsourcing drug discovery services, growing life sciences research, and increasing government initiatives for healthcare research.
One significant growth driver for the viral vector and plasmid DNA manufacturing market in the Asia Pacific region is the increasing investment in healthcare infrastructure and research & development (R&D) activities. Governments and private entities are allocating substantial funds and launching various research programs to enhance healthcare facilities and promote scientific advancements, including viral vector and plasmid DNA manufacturing technologies. These investments are driving the adoption of viral vector and plasmid DNA manufacturing techniques across various applications and research fields.
The Asia-Pacific Economic Cooperation (APEC) Life Sciences Innovation Forum (LSIF) and its Regulatory Harmonization Steering Committee (RHSC) adopted a strategic plan (Vision 2020: A Strategic Framework: Regulatory Convergence for Medical Products by 2020). This strategic plan provided the basic proposal and rationale for achieving regional regulatory convergence of medical product approval procedure. The forum’s vision is to accelerate regulatory convergence for medical products in the APEC region as much as possible by 2030 in order to protect people’s safety, make life-saving products available, save public resources, attract investments, mitigate corruption, and improve global standing in every APEC economy. Thus, favourable regulatory guidelines for product approval is expected to drive the market growth.
To know about the assumptions considered for the study, download the pdf brochure
Key players in the viral vector and plasmid DNA manufacturing market include Lonza Group AG (Switzerland), Merck KGaA (Germany), Thermo Fisher Scientific Inc. (US), Charles River Laboratories International, Inc. (US), Catalent Inc. (US), WuXi AppTec (China), FUJIFILM Corporation (Japan), GenScript Biotech Corporation (US), Takara Bio Inc. (Japan), Oxford Biomedica (UK), Novartis AG (Switzerland), Precision Biosciences (US), Bluebird Bio, Inc. (US), Sartorius AG (Germany), Danaher Corporation (US), SIRON Biotech (Germany), VGXI, Inc. (US), Waisman Biomanufacturing (US), Kaneka Eurogentec S.A. (Belgium), PlasmidFactory GmbH (Germany), ATUM (US), Addgene (US), Cell and Gene Therapy Catapult (UK), Batavia biosciences (Netherlands), and Altogen Biosystems (US).
Viral Vector & Plasmid DNA Manufacturing Market Report Scope
|
Report Metric |
Details |
|
Market Revenue in 2023 |
$5.5 billion |
|
Projected Revenue by 2028 |
$12.8 billion |
|
Revenue Rate |
poised to grow at a CAGR of 18.2% |
|
Growth Driver |
Effectiveness of viral vectors |
|
Growth Opportunity |
Leveraging digital to facilitate operational excellence |
This report categorizes the viral vector and plasmid DNA manufacturing market to forecast revenue and analyze trends in each of the following submarkets:
By Type
-
Viral Vectors
- Retroviruses
- Adenoviruses
- Adeno-associated viruses (AAVs)
- Lentiviruses
- Others
- Plasmid DNA
By Product & Services
- Product
- Services
By Workflow
-
Upstream Manufacturing
- Vector Amplification, Editing and Expansion
- Vector Recovery/Harvesting
-
Downstream Manufacturing
- Purification
- Fill Finish
By Application
- Cell and Gene Therapy
- Vaccine Development
- Research
By Disease Indication
- Cancer
- Generic Disorders
- Infectious Diseases
- Others
By End User
- Pharmaceutical and Biopharmaceutical Companies
- Academics and Research Institutes
- Others
By Region
-
North America
- US
- Canada
-
Europe
- UK
- Germany
- France
- Italy
- Spain
- Rest of Europe (RoE)
-
Asia Pacific
- China
- Japan
- India
- South Korea
- Rest of Asia Pacific (RoAPAC)
-
Latin America (LATAM)
- Brazil
- RoLATAM
- Middle East and Africa (MEA)
Recent Developments
- In August 2022, MERCK KGaA the VirusExpress 293 Adeno-Associated Virus (AAV) Production Platform, which offers a full viral vector manufacturing offering including AAV, Lentiviral vectors.
- In May 2022, Catalent Inc., launched UpTempo Virtuoso platform process for the development and manufacturing of adeno-associated viral (AAV) vectors.
Frequently Asked Questions (FAQ):
What is the projected market revenue value of the global viral vector manufacturing market?
The global viral vector manufacturing market boasts a total revenue value of $12.8 billion by 2028.
What is the estimated growth rate (CAGR) of the global viral vector manufacturing market?
The global viral vector manufacturing market has an estimated compound annual growth rate (CAGR) of 18.2% and a revenue size in the region of $5.5 billion in 2023.
To speak to our analyst for a discussion on the above findings, click Speak to Analyst


- 5.1 INTRODUCTION
-
5.2 MARKET DYNAMICSDRIVERS- Rising prevalence of genetic disorders, cancer, and infectious diseases- Availability of funding for development of gene therapy- Effectiveness of viral vectors- Ongoing research on viral vector-based gene and cell therapiesRESTRAINTS- High operational costs associated with cell and gene therapy manufacturing- Short shelf life of viral vectorsOPPORTUNITIES- Smart capital deployment and planning for scalability- Leveraging digital tools to facilitate operational excellenceCHALLENGES- Risk of mutagenesis and other unwanted outcomes- Individual optimization and low yield
- 5.3 INDICATIVE PRICING ANALYSIS
- 5.4 VALUE CHAIN ANALYSIS
-
5.5 VALUE CHAIN ANALYSIS: MANUFACTURING PHASES ADD MAXIMUM VALUEECOSYSTEM ANALYSIS
-
5.6 PORTER’S FIVE FORCES ANALYSISTHREAT OF NEW ENTRANTSTHREAT OF SUBSTITUTESBARGAINING POWER OF BUYERSBARGAINING POWER OF SUPPLIERSINTENSITY OF COMPETITIVE RIVALRY
- 5.7 TECHNOLOGY ANALYSIS
-
5.8 PATENT ANALYSIS
-
5.9 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS’ BUSINESSES
-
5.10 REGULATORY ANALYSISREGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.11 KEY CONFERENCES AND EVENTS IN 2022–2023
-
5.12 KEY STAKEHOLDERS AND BUYING CRITERIAKEY STAKEHOLDERS IN BUYING PROCESSBUYING CRITERIA FOR VIRAL VECTOR AND PLASMID DNA MANUFACTURING PRODUCTS AND SERVICES
- 6.1 INTRODUCTION
-
6.2 VIRAL VECTORRETROVIRUS- Rising application of retroviruses in gene therapy and gene transfer studies to support growthADENOVIRUS- Large capacity for carrying genetic material to promote growthADENO-ASSOCIATED VIRUS- Low toxicity to support demand growthLENTIVIRUS- Ability to infect both dividing and non-dividing cells to drive growthOTHER VIRAL VECTORS
-
6.3 PLASMID DNAGROWING R&D AND LAUNCHES OF PRODUCTION PLATFORMS TO BOOST MARKET
- 7.1 INTRODUCTION
-
7.2 PRODUCTSINCREASING PRODUCT LAUNCHES TO DRIVE MARKET GROWTH
-
7.3 SERVICESINCREASING DEMAND FOR SERVICES TO BOOST MARKET
- 8.1 INTRODUCTION
-
8.2 UPSTREAM MANUFACTURINGVECTOR AMPLIFICATION, EDITING, AND EXPANSION- Increasing demand for gene therapy to fuel growthVECTOR RECOVERY/HARVESTING- Rising clinical trials and approvals of cell and gene-based therapies to drive demand for vector recovery/harvesting
-
8.3 DOWNSTREAM MANUFACTURINGPURIFICATION- Technological advancements in purification to drive marketFILL-FINISH- Technological advancements in downstream manufacturing process
- 9.1 INTRODUCTION
-
9.2 CELL & GENE THERAPYROBUST PIPELINE FOR CELL & GENE THERAPY TO DRIVE MARKET
-
9.3 VACCINE DEVELOPMENTDEVELOPMENT OF NOVEL VACCINES TO DRIVE DEMAND FOR VIRAL VECTORS AND PLASMID DNA
-
9.4 RESEARCHRISING R&D TO INTRODUCE NEW APPLICATIONS OF VIRAL VECTOR AND PLASMID DNA
- 10.1 INTRODUCTION
-
10.2 CANCERINCREASING PATIENT POPULATION TO PROPEL MARKET GROWTH
-
10.3 GENETIC DISORDERSLAUNCH OF ADVANCED TOOLS TO DELIVER THERAPEUTIC GENES TO SUPPORT MARKET GROWTH
-
10.4 INFECTIOUS DISEASESINCREASING PREVALENCE OF INFECTIOUS DISEASES TO DRIVE MARKET
- 10.5 OTHER DISEASE INDICATIONS
- 11.1 INTRODUCTION
-
11.2 PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIESGROWING CELL & GENE THERAPY R&D TO BOOST GROWTH
-
11.3 ACADEMIC & RESEARCH INSTITUTESINCREASING DRUG DISCOVERY RESEARCH PROGRAMS TO DRIVE MARKET
- 11.4 OTHER END USERS
- 12.1 INTRODUCTION
-
12.2 NORTH AMERICANORTH AMERICA: RECESSION IMPACTUS- Increasing incidence of cancer to propel marketCANADA- Government initiatives to promote research to support market growth
-
12.3 EUROPEEUROPE: RECESSION IMPACTGERMANY- Presence of large number of academic research institutes to propel marketUK- Increasing R&D investments by pharmaceutical companies to support marketFRANCE- Focus on increasing use of generic drugs to drive marketITALY- Collaborative research initiatives to support market growthSPAIN- Rising R&D expenditure to boost market growthREST OF EUROPE
-
12.4 ASIA PACIFICASIA PACIFIC: RECESSION IMPACTCHINA- Low manufacturing costs and high demand for medicines to drive marketJAPAN- Growing geriatric population to support market growthINDIA- Growing pharmaceutical industry to create favorable opportunities for market playersSOUTH KOREA- Strong foundation for development and production of viral vectors to support market growthREST OF ASIA PACIFIC
-
12.5 LATIN AMERICALATIN AMERICA: RECESSION IMPACTBRAZIL- Increasing pharmaceutical R&D to drive marketREST OF LATIN AMERICA
-
12.6 MIDDLE EAST & AFRICAGROWING PHARMACEUTICAL INDUSTRY TO DRIVE MARKETMIDDLE EAST & AFRICA: RECESSION IMPACT

- 13.1 INTRODUCTION
- 13.2 RIGHT-TO-WIN APPROACHES ADOPTED BY KEY PLAYERS
- 13.3 MARKET SHARE ANALYSIS
- 13.4 REVENUE SHARE ANALYSIS
-
13.5 COMPANY EVALUATION MATRIX: KEY PLAYERSSTARSEMERGING LEADERSPERVASIVE PLAYERSPARTICIPANTS
-
13.6 COMPETITIVE BENCHMARKING OF TOP 25 PLAYERSPRODUCT & SERVICE FOOTPRINT OF COMPANIES (25 COMPANIES)REGIONAL FOOTPRINT OF COMPANIES (25 COMPANIES)
-
13.7 COMPANY EVALUATION MATRIX: START-UPS/SMESPROGRESSIVE COMPANIESSTARTING BLOCKSRESPONSIVE COMPANIESDYNAMIC COMPANIES
- 13.8 COMPETITIVE BENCHMARKING OF START-UP/SME PLAYERS
-
13.9 COMPETITIVE SCENARIO AND TRENDSPRODUCT & SERVICE LAUNCHESDEALSOTHER DEVELOPMENTS
-
14.1 KEY COMPANIESLONZA GROUP- Business overview- Products & services offered- Recent developments- MnM viewMERCK KGAA- Business overview- Products & services offered- Recent developments- MnM viewTHERMO FISHER SCIENTIFIC INC.- Business overview- Products & services offered- Recent developments- MnM viewCHARLES RIVER LABORATORIES INTERNATIONAL, INC.- Business overview- Products & services offered- Recent developmentsCATALENT, INC.- Business overview- Products & services offered- Recent developmentsWUXI APPTEC CO., LTD.- Business overview- Products & services offered- Recent developmentsFUJIFILM CORPORATION- Business overview- Products & services offered- Recent developmentsGENSCRIPT BIOTECH CORPORATION- Business overview- Products & services offered- Recent developmentsTAKARA BIO INC.- Business overview- Products & services offered- Recent developmentsOXFORD BIOMEDICA- Business overview- Products & services offered- Recent developmentsNOVARTIS AG- Business overview- Products & services offered- Recent developmentsPRECISION BIOSCIENCES- Business overview- Products & services offered- Recent developmentsBLUEBIRD BIO, INC.- Business overview- Products & services offeredSARTORIUS AG- Business overview- Products & services offered- Recent developmentsDANAHER CORPORATION- Business overview- Products & services offered- Recent developmentsSIRION BIOTECH- Business overview- Products & services offered- Recent developments
-
14.2 OTHER PLAYERSVGXI, INC.WAISMAN BIOMANUFACTURINGKANEKA EUROGENTEC S.A.PLASMIDFACTORY GMBHATUMADDGENECELL AND GENE THERAPY CATAPULTBATAVIA BIOSCIENCES B.V.ALTOGEN BIOSYSTEMS
- 15.1 DISCUSSION GUIDE
- 15.2 KNOWLEDGESTORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
- 15.3 CUSTOMIZATION OPTIONS
- 15.4 RELATED REPORTS
- 15.5 AUTHOR DETAILS
- TABLE 1 GLOBAL INFLATION RATE PROJECTIONS, 2021–2027 (% GROWTH)
- TABLE 2 US HEALTH EXPENDITURE, 2019–2022 (USD MILLION)
- TABLE 3 US HEALTH EXPENDITURE, 2023–2027 (USD MILLION)
- TABLE 4 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET: IMPACT ANALYSIS OF DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- TABLE 5 OVERVIEW OF CURRENT CLINICAL TRIALS BASED ON VECTOR TYPE
- TABLE 6 PRICING ANALYSIS FOR PRODUCTS
- TABLE 7 OVERALL MARKET ECOSYSTEM FOR VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET
- TABLE 8 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET: PORTER’S FIVE FORCES ANALYSIS
- TABLE 9 EXAMPLES OF ADVANCED PRODUCTS/PLATFORMS
- TABLE 10 EXAMPLES OF RECENT PATENT ACTIVITY IN VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET
- TABLE 11 NORTH AMERICA: LIST OF REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 12 EUROPE: LIST OF REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 13 ASIA PACIFIC: LIST OF REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 14 LATIN AMERICA: LIST OF REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 15 MIDDLE EAST & AFRICA: LIST OF REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 16 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET: LIST OF CONFERENCES AND EVENTS
- TABLE 17 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 18 VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 19 VIRAL VECTOR MANUFACTURING MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 20 NORTH AMERICA: VIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 21 EUROPE: VIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 22 ASIA PACIFIC: VIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 23 LATIN AMERICA: VIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 24 RETROVIRAL VECTOR MANUFACTURING MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 25 NORTH AMERICA: RETROVIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 26 EUROPE: RETROVIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 27 ASIA PACIFIC: RETROVIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 28 LATIN AMERICA: RETROVIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 29 ADENOVIRAL VECTOR MANUFACTURING MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 30 NORTH AMERICA: ADENOVIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 31 EUROPE: ADENOVIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 32 ASIA PACIFIC: ADENOVIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 33 LATIN AMERICA: ADENOVIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 34 AAV VECTOR MANUFACTURING MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 35 NORTH AMERICA: AAV VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 36 EUROPE: AAV VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 37 ASIA PACIFIC: AAV VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 38 LATIN AMERICA: AAV VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 39 LENTIVIRAL VECTOR MANUFACTURING MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 40 NORTH AMERICA: LENTIVIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 41 EUROPE: LENTIVIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 42 ASIA PACIFIC: LENTIVIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 43 LATIN AMERICA: LENTIVIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 44 OTHER VIRAL VECTOR MANUFACTURING MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 45 NORTH AMERICA: OTHER VIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 46 EUROPE: OTHER VIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 47 ASIA PACIFIC: OTHER VIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 48 LATIN AMERICA: OTHER VIRAL VECTOR MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 49 PLASMID DNA MANUFACTURING MARKET, BY REGION 2021–2028 (USD MILLION)
- TABLE 50 NORTH AMERICA: PLASMID DNA MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 51 EUROPE: PLASMID DNA MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 52 ASIA PACIFIC: PLASMID DNA MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 53 LATIN AMERICA: PLASMID DNA MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 54 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 55 VIRAL VECTOR AND PLASMID DNA MANUFACTURING PRODUCTS, BY KEY PLAYER
- TABLE 56 VIRAL VECTOR AND PLASMID DNA MANUFACTURING PRODUCTS MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 57 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING PRODUCTS MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 58 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING PRODUCTS MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 59 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING PRODUCTS MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 60 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING PRODUCTS MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 61 VIRAL VECTOR AND PLASMID DNA MANUFACTURING SERVICES, BY KEY PLAYER
- TABLE 62 VIRAL VECTOR AND PLASMID DNA MANUFACTURING SERVICES MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 63 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING SERVICES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 64 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING SERVICES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 65 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING SERVICES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 66 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING SERVICES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 67 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 68 VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 69 VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 70 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 71 EUROPE: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 72 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 73 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 74 VECTOR AMPLIFICATION, EDITING, AND EXPANSION PROCESSES MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 75 NORTH AMERICA: VECTOR AMPLIFICATION, EDITING, AND EXPANSION PROCESSES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 76 EUROPE: VECTOR AMPLIFICATION, EDITING, AND EXPANSION PROCESSES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 77 ASIA PACIFIC: VECTOR AMPLIFICATION, EDITING, AND EXPANSION PROCESSES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 78 LATIN AMERICA: VECTOR AMPLIFICATION, EDITING, AND EXPANSION PROCESSES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 79 VECTOR RECOVERY/HARVESTING PROCESSES MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 80 NORTH AMERICA: VECTOR RECOVERY/HARVESTING PROCESSES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 81 EUROPE: VECTOR RECOVERY/HARVESTING PROCESSES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 82 ASIA PACIFIC: VECTOR RECOVERY/HARVESTING PROCESSES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 83 LATIN AMERICA: VECTOR RECOVERY/HARVESTING PROCESSES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 84 VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 85 VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY REGION 2021–2028 (USD MILLION)
- TABLE 86 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 87 EUROPE: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 88 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 89 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 90 PURIFICATION PROCESSES MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 91 NORTH AMERICA: PURIFICATION PROCESSES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 92 EUROPE: PURIFICATION PROCESSES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 93 ASIA PACIFIC: PURIFICATION PROCESSES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 94 LATIN AMERICA: PURIFICATION PROCESSES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 95 FILL-FINISH PROCESSES MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 96 NORTH AMERICA: FILL-FINISH PROCESSES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 97 EUROPE: FILL-FINISH PROCESSES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 98 ASIA PACIFIC: FILL-FINISH PROCESSES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 99 LATIN AMERICA: FILL-FINISH PROCESSES MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 100 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 101 NUMBER OF CELL AND GENE THERAPIES IN CLINICAL TRIAL PHASES (2021)
- TABLE 102 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR CELL & GENE THERAPY, BY REGION, 2021–2028 (USD MILLION)
- TABLE 103 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR CELL & GENE THERAPY, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 104 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR CELL & GENE THERAPY, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 105 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR CELL & GENE THERAPY, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 106 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR CELL & GENE THERAPY, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 107 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR VACCINE DEVELOPMENT, BY REGION 2021–2028 (USD MILLION)
- TABLE 108 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR VACCINE DEVELOPMENT, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 109 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR VACCINE DEVELOPMENT, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 110 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR VACCINE DEVELOPMENT, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 111 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR VACCINE DEVELOPMENT, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 112 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR RESEARCH, BY REGION 2021–2028 (USD MILLION)
- TABLE 113 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR RESEARCH, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 114 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR RESEARCH, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 115 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR RESEARCH, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 116 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR RESEARCH, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 117 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 118 NUMBER OF NEW CANCER CASES, BY TYPE, 2020 VS. 2040
- TABLE 119 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR CANCER, BY REGION, 2021–2028 (USD MILLION)
- TABLE 120 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR CANCER, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 121 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR CANCER, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 122 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR CANCER, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 123 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR CANCER, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 124 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR GENETIC DISORDERS, BY REGION, 2021–2028 (USD MILLION)
- TABLE 125 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR GENETIC DISORDERS, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 126 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR GENETIC DISORDERS, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 127 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR GENETIC DISORDERS, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 128 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR GENETIC DISORDERS, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 129 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR INFECTIOUS DISEASES, BY REGION, 2021–2028 (USD MILLION)
- TABLE 130 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR INFECTIOUS DISEASES, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 131 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR INFECTIOUS DISEASES, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 132 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR INFECTIOUS DISEASES, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 133 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR INFECTIOUS DISEASES, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 134 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR OTHER DISEASE INDICATIONS, BY REGION, 2021–2028 (USD MILLION)
- TABLE 135 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR OTHER DISEASE INDICATIONS, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 136 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR OTHER DISEASE INDICATIONS, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 137 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR OTHER DISEASE INDICATIONS, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 138 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR OTHER DISEASE INDICATIONS, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 139 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 140 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIES, BY REGION, 2021–2028 (USD MILLION)
- TABLE 141 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIES, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 142 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIES, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 143 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIES, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 144 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIES, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 145 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY REGION, 2021–2028 (USD MILLION)
- TABLE 146 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 147 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 148 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 149 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 150 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR OTHER END USERS, BY REGION, 2021–2028 (USD MILLION)
- TABLE 151 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR OTHER END USERS, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 152 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR OTHER END USERS, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 153 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR OTHER END USERS, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 154 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET FOR OTHER END USERS, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 155 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 156 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 157 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 158 NORTH AMERICA: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 159 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 160 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 161 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 162 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 163 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 164 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 165 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 166 US: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 167 US: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 168 US: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 169 US: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 170 US: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 171 US: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 172 US: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 173 US: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 174 US: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 175 CANADA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 176 CANADA: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 177 CANADA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 178 CANADA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 179 CANADA: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 180 CANADA: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 181 CANADA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 182 CANADA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 183 CANADA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 184 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 185 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 186 EUROPE: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 187 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 188 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 189 EUROPE: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 190 EUROPE: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 191 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 192 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 193 EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 194 GERMANY: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 195 GERMANY: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 196 GERMANY: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 197 GERMANY: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 198 GERMANY: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 199 GERMANY: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 200 GERMANY: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 201 GERMANY: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 202 GERMANY: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 203 UK: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 204 UK: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 205 UK: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 206 UK: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 207 UK: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 208 UK: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 209 UK: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 210 UK: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 211 UK: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 212 FRANCE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 213 FRANCE: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 214 FRANCE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 215 FRANCE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 216 FRANCE: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 217 FRANCE: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 218 FRANCE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 219 FRANCE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 220 FRANCE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 221 ITALY: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 222 ITALY: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 223 ITALY: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 224 ITALY: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 225 ITALY: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 226 ITALY: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 227 ITALY: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 228 ITALY: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 229 ITALY: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 230 SPAIN: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 231 SPAIN: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 232 SPAIN: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 233 SPAIN: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 234 SPAIN: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 235 SPAIN: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 236 SPAIN: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 237 SPAIN: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 238 SPAIN: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 239 REST OF EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 240 REST OF EUROPE: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 241 REST OF EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 242 REST OF EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 243 REST OF EUROPE: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 244 REST OF EUROPE: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 245 REST OF EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 246 REST OF EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 247 REST OF EUROPE: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 248 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 249 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 250 ASIA PACIFIC: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 251 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 252 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 253 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 254 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 255 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 256 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 257 ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 258 CHINA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 259 CHINA: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 260 CHINA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 261 CHINA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 262 CHINA: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 263 CHINA: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 264 CHINA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 265 CHINA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 266 CHINA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 267 JAPAN: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 268 JAPAN: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 269 JAPAN: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 270 JAPAN: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 271 JAPAN: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 272 JAPAN: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 273 JAPAN: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 274 JAPAN: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 275 JAPAN: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 276 INDIA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 277 INDIA: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 278 INDIA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 279 INDIA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 280 INDIA: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 281 INDIA: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 282 INDIA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 283 INDIA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 284 INDIA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 285 SOUTH KOREA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 286 SOUTH KOREA: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 287 SOUTH KOREA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 288 SOUTH KOREA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 289 SOUTH KOREA: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, 2021–2028 (USD MILLION)
- TABLE 290 SOUTH KOREA: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 291 SOUTH KOREA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 292 SOUTH KOREA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 293 SOUTH KOREA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 294 REST OF ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 295 REST OF ASIA PACIFIC: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 296 REST OF ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 297 REST OF ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 298 REST OF ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 299 REST OF ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 300 REST OF ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 301 REST OF ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 302 REST OF ASIA PACIFIC: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 303 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 304 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 305 LATIN AMERICA: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 306 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 307 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 308 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 309 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 310 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 311 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 312 LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 313 BRAZIL: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 314 BRAZIL: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 315 BRAZIL: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 316 BRAZIL: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 317 BRAZIL: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 318 BRAZIL: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 319 BRAZIL: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 320 BRAZIL: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 321 BRAZIL: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 322 REST OF LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 323 REST OF LATIN AMERICA: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 324 REST OF LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 325 REST OF LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 326 REST OF LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 327 REST OF LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 328 REST OF LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 329 REST OF LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 330 REST OF LATIN AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 331 MIDDLE EAST & AFRICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 332 MIDDLE EAST & AFRICA: VIRAL VECTOR MANUFACTURING MARKET, BY TYPE, 2021–2028 (USD MILLION)
- TABLE 333 MIDDLE EAST & AFRICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2021–2028 (USD MILLION)
- TABLE 334 MIDDLE EAST & AFRICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2021–2028 (USD MILLION)
- TABLE 335 MIDDLE EAST & AFRICA: VIRAL VECTOR AND PLASMID DNA UPSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 336 MIDDLE EAST & AFRICA: VIRAL VECTOR AND PLASMID DNA DOWNSTREAM MANUFACTURING MARKET, BY PROCESS, 2021–2028 (USD MILLION)
- TABLE 337 MIDDLE EAST & AFRICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 338 MIDDLE EAST & AFRICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2021–2028 (USD MILLION)
- TABLE 339 MIDDLE EAST & AFRICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 340 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET: DEGREE OF COMPETITION
- TABLE 341 PRODUCT & SERVICE FOOTPRINT ANALYSIS OF KEY PLAYERS IN VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET
- TABLE 342 REGIONAL FOOTPRINT ANALYSIS OF KEY PLAYERS IN VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET
- TABLE 343 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET: DETAILED LIST OF KEY START-UP/SME PLAYERS
- TABLE 344 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET: COMPETITIVE BENCHMARKING OF START-UP/SME PLAYERS
- TABLE 345 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET: PRODUCT & SERVICE LAUNCHES, JANUARY 2020–MAY 2023
- TABLE 346 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET: DEALS, JANUARY 2020–MAY 2023
- TABLE 347 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET: OTHER DEVELOPMENTS, JANUARY 2020–MAY 2023
- TABLE 348 LONZA GROUP: BUSINESS OVERVIEW
- TABLE 349 MERCK KGAA: BUSINESS OVERVIEW
- TABLE 350 THERMO FISHER SCIENTIFIC INC.: BUSINESS OVERVIEW
- TABLE 351 CHARLES RIVER LABORATORIES INTERNATIONAL, INC.: BUSINESS OVERVIEW
- TABLE 352 CATALENT, INC: BUSINESS OVERVIEW
- TABLE 353 WUXI APPTEC CO., LTD.: BUSINESS OVERVIEW
- TABLE 354 FUJIFILM CORPORATION: BUSINESS OVERVIEW
- TABLE 355 GENSCRIPT BIOTECH CORPORATION: BUSINESS OVERVIEW
- TABLE 356 TAKARA BIO INC.: BUSINESS OVERVIEW
- TABLE 357 OXFORD BIOMEDICA: BUSINESS OVERVIEW
- TABLE 358 NOVARTIS AG: BUSINESS OVERVIEW
- TABLE 359 PRECISION BIOSCIENCES: BUSINESS OVERVIEW
- TABLE 360 BLUEBIRD BIO, INC.: BUSINESS OVERVIEW
- TABLE 361 SARTORIUS AG: BUSINESS OVERVIEW
- TABLE 362 DANAHER CORPORATION: BUSINESS OVERVIEW
- TABLE 363 SIRION BIOTECH: BUSINESS OVERVIEW
- FIGURE 1 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET SEGMENTATION
- FIGURE 2 RESEARCH DESIGN
- FIGURE 3 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET: BREAKDOWN OF PRIMARIES
- FIGURE 4 MARKET SIZE ESTIMATION: SUPPLY-SIDE ANALYSIS, 2022
- FIGURE 5 MARKET SIZE ESTIMATION: APPROACH 1 (REVENUE SHARE ANALYSIS), 2022
- FIGURE 6 ILLUSTRATIVE EXAMPLE OF LONZA GROUP AG: REVENUE SHARE ANALYSIS (2022)
- FIGURE 7 MARKET VALIDATION FROM PRIMARY EXPERTS
- FIGURE 8 MARKET SIZE ESTIMATION METHODOLOGY: TOP-DOWN APPROACH
- FIGURE 9 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET: CAGR PROJECTIONS
- FIGURE 10 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET: GROWTH ANALYSIS OF DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- FIGURE 11 DATA TRIANGULATION METHODOLOGY
- FIGURE 12 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY TYPE, 2023 VS. 2028 (USD MILLION)
- FIGURE 13 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY PRODUCT & SERVICE, 2023 VS. 2028 (USD MILLION)
- FIGURE 14 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY WORKFLOW, 2023 VS. 2028 (USD MILLION)
- FIGURE 15 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY APPLICATION, 2023 VS. 2028 (USD MILLION)
- FIGURE 16 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY DISEASE INDICATION, 2023 VS. 2028 (USD MILLION)
- FIGURE 17 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, BY END USER, 2023 VS. 2028 (USD MILLION)
- FIGURE 18 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET: GEOGRAPHICAL SNAPSHOT
- FIGURE 19 ONGOING RESEARCH ON VIRAL VECTOR-BASED CELL AND GENE THERAPIES TO DRIVE MARKET
- FIGURE 20 PRODUCTS SEGMENT IN US ACCOUNTED FOR LARGEST MARKET SHARE IN 2022
- FIGURE 21 PRODUCTS SEGMENT TO WITNESS HIGHEST GROWTH RATE DURING FORECAST PERIOD
- FIGURE 22 PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIES SEGMENT ACCOUNTED FOR LARGEST MARKET SHARE IN 2022
- FIGURE 23 CHINA TO REGISTER HIGHEST CAGR DURING FORECAST PERIOD
- FIGURE 24 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- FIGURE 25 ECOSYSTEM ANALYSIS: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET
- FIGURE 26 TOP PATENT OWNERS IN VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET, JANUARY 2014 TO JANUARY 2023
- FIGURE 27 REVENUE SHIFT AND NEW REVENUE POCKETS FOR VIRAL VECTOR AND PLASMID DNA MANUFACTURERS
- FIGURE 28 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS OF VIRAL VECTOR AND PLASMID DNA MANUFACTURING PRODUCTS
- FIGURE 29 KEY BUYING CRITERIA FOR END USERS
- FIGURE 30 NORTH AMERICA: VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET SNAPSHOT
- FIGURE 31 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET: STRATEGIES ADOPTED BY KEY PLAYERS, 2020–2023
- FIGURE 32 MARKET SHARE ANALYSIS OF KEY PLAYERS, 2022
- FIGURE 33 REVENUE ANALYSIS OF KEY PLAYERS, 2020–2022
- FIGURE 34 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET: COMPANY EVALUATION MATRIX FOR KEY PLAYERS, 2022
- FIGURE 35 VIRAL VECTOR AND PLASMID DNA MANUFACTURING MARKET: COMPANY EVALUATION MATRIX FOR START-UPS/SMES, 2022
- FIGURE 36 LONZA GROUP: COMPANY SNAPSHOT (2022)
- FIGURE 37 MERCK KGAA: COMPANY SNAPSHOT (2022)
- FIGURE 38 THERMO FISHER SCIENTIFIC INC.: COMPANY SNAPSHOT (2022)
- FIGURE 39 CHARLES RIVER LABORATORIES INTERNATIONAL, INC.: COMPANY SNAPSHOT (2022)
- FIGURE 40 CATALENT, INC: COMPANY SNAPSHOT (2022)
- FIGURE 41 WUXI APPTEC CO., LTD.: COMPANY SNAPSHOT (2022)
- FIGURE 42 FUJIFILM CORPORATION: COMPANY SNAPSHOT (2022)
- FIGURE 43 GENSCRIPT BIOTECH CORPORATION: COMPANY SNAPSHOT (2021)
- FIGURE 44 TAKARA BIO INC.: COMPANY SNAPSHOT (2022)
- FIGURE 45 OXFORD BIOMEDICA: COMPANY SNAPSHOT (2022)
- FIGURE 46 NOVARTIS AG: COMPANY SNAPSHOT (2022)
- FIGURE 47 PRECISION BIOSCIENCES: COMPANY SNAPSHOT (2022)
- FIGURE 48 BLUEBIRD BIO, INC.: COMPANY SNAPSHOT (2022)
- FIGURE 49 SARTORIUS AG: COMPANY SNAPSHOT (2022)
- FIGURE 50 DANAHER CORPORATION: COMPANY SNAPSHOT (2022)
This research study involved the extensive use of secondary sources, directories, and databases to identify and collect valuable information for the analysis of the global viral vector and plasmid DNA manufacturing market. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess growth prospects of the market. The global market size estimated through secondary research was then triangulated with inputs from primary research to arrive at the final market size.
Secondary Research
The secondary sources referred to for this research study include publications from government sources, such as American Society of Gene & Cell Therapy (ASGCT), Centers for Disease Control and Prevention (CDC), GLOBOCAN, World Health Organization (WHO), Food and Drug Administration (FDA), National Institutes of Health (NIH), International Society for Cell & Gene Therapy (ISCT), European Society of Gene and Cell Therapy, Japan Human Cell Society, Pharmaceutical Research and Manufacturers of America (PhRMA), European Federation of Pharmaceutical Industries and Associations (EFPIA), PubMed, Government Associations. Secondary sources also include corporate and regulatory filings, such as annual reports, SEC filings, investor presentations, and financial statements; business magazines & research journals; press releases; and trade, business, and professional associations. Secondary data was collected and analyzed to arrive at the overall size of the global viral vector and plasmid DNA manufacturing market, which was validated through primary research.
Primary Research
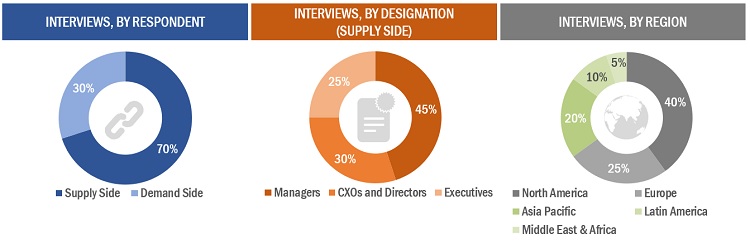
Extensive primary research was conducted after acquiring basic knowledge about the global viral vector and plasmid DNA manufacturing market scenario through secondary research. Several primary interviews were conducted with market experts from the demand side, such as personnel from pharmaceutical and biopharmaceutical industries, CMOs and CROs, academic & research institutes, and experts from the supply side, such as C-level and D-level executives, product managers, marketing & sales managers of key manufacturers, distributors, and channel partners. These interviews were conducted across four major regions, including the Asia Pacific, North America, Europe, Latin America, and the Middle East & Africa. Approximately 70% and 30% of the primary interviews were conducted with supply-side and demand-side participants, respectively. This primary data was collected through questionnaires, e-mails, online surveys, personal interviews, and telephonic interviews.
The following is a breakdown of the primary respondents:
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
The global size of the market was estimated through multiple approaches. A detailed market estimation approach was followed to estimate and validate the value of the viral vector and plasmid DNA manufacturing market and other dependent submarkets. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
- The key players in the industry and market have been identified through extensive primary and secondary research.
- The revenues generated from the viral vector and plasmid DNA manufacturing business of leading players have been determined through primary and secondary research.
- All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources.
Data Triangulation
After arriving at the market size from the market size estimation process explained above, the total market was divided into several segments and subsegments. Data triangulation and market breakdown procedures were employed, wherever applicable, to complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments.
Market Definition
Viral vector and plasmid DNA manufacturing market includes the products and services used for development and manufacturing of viral vector and plasmid DNA. Viral vectors are used to deliver genetic material into cells, while plasmid DNA is used to produce proteins or other molecules that can be used as vaccines or therapeutics. These technologies are being increasingly used to develop treatments for a range of diseases, including cancer, genetic disorders, and infectious diseases.
Key Stakeholders
- Products and services provider for viral vector and plasmid DNA manufacturing
- Suppliers and distributors of viral vector and plasmid DNA manufacturing products
- Research institutes and academic centers
- Pharmaceutical & biopharmaceutical companies
- Contract research organizations (CROs)
- Contract development and manufacturing organizations (CDMOs)
- Molecular diagnostic companies
- Business research and consulting service providers
- Venture capitalists
- Regulatory bodies
- Government bodies
Report Objectives
- To define, describe, and forecast the global Viral vector and plasmid DNA manufacturing market based on type, product & service, workflow, application, disease indication, end user, and region
- To provide detailed information regarding the major factors influencing the growth of the market (such as drivers, restraints, opportunities, and challenges)
- To strategically analyze micromarkets with respect to individual growth trends, prospects, and contributions to the overall market
- To analyze the opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders
- To forecast the size of the market segments with respect to five main regions, namely, North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa
- To profile the key players and comprehensively analyze their product & service portfolios, market positions, and core competencies
- To track and analyze competitive developments such as acquisitions, product/ service launches, expansions, agreements, partnerships, and collaborations in the market
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for this report:
Geographical Analysis
- Further breakdown of the Rest of Europe viral vector and plasmid DNA manufacturing market, by country
- Further breakdown of the Rest of Asia Pacific viral vector and plasmid DNA manufacturing market, by country
- Further breakdown of the Rest of Latin America and Middle East & Africa viral vector and plasmid DNA manufacturing market, by country
Company Information
- Detailed analysis and profiling of additional market players (up to five)
Segment Analysis
- Further breakdown of the disease indication area segment as per the service portfolio of prominent players operating in the market.



 Generating Response ...
Generating Response ...











Growth opportunities and latent adjacency in Viral Vector & Plasmid DNA Manufacturing Market